Scientists have successfully transplanted clusters of human neurons into the
brains of newborn rats, a striking feat of biological engineering that may
provide more realistic models for neurological conditions such as autism and
serve as a way to restore injured brains.
اضافة اعلان
In a study
published earlier this month, researchers from
Stanford University reported
that the clumps of human cells, known as “organoids”, grew into millions of new
neurons and wired themselves into their new nervous systems. Once the organoids
had plugged into the brains of the rats, the animals could receive sensory
signals from their whiskers and help generate command signals to guide their
movements.
Dr Sergiu Pasca,
the neuroscientist who led the research, said he and his colleagues were now
using the transplanted neurons to learn about the biology underlying autism,
schizophrenia, and other developmental disorders.
“If we really want
to tackle the biology of these conditions, we’re going to need more complex
models of the human brain,” Pasca said.
In 2009, after
training in medicine in Romania, Pasca joined Stanford as a postdoctoral
researcher to learn how to create human neurons in a dish. He and his
colleagues took skin cells from volunteers and bathed them in chemicals that
caused them to change character. Now they were more like embryo cells, which
can become any tissue in the body.
With the addition
of more chemicals, the researchers coaxed the cells to develop into neurons.
They could then observe pulses of voltage shoot down the length of the neurons
as they lay in a dish.
 Dr Sergiu Pasca, a neuroscientist at Stanford University.
Dr Sergiu Pasca, a neuroscientist at Stanford University.
Pasca and his colleagues
carried out the same experiment again, this time using skin cells from people
with Timothy syndrome, a rare form of autism caused by a single mutation that
leads to serious heart problems as well as impaired language and social skills.
Growing
Timothy syndrome neurons in a dish, Pasca could see a number of differences between
them and typical neurons. They produced extra amounts of signaling chemicals
such as dopamine, for example.
But examining
single cells could reveal only a limited number of clues about the condition.
Pasca suspected that he could learn more by studying thousands of neurons
joined together in circuits called brain organoids.
A new chemical
recipe allowed Pasca to mimic the condition inside the developing brain. Bathed
in this broth, skin cells turned into progenitor brain cells, which in turn
became tangles of neurons found in the brain’s outer layers, called the cortex.
In a later study,
he and his colleagues connected three organoids: one made of cortex, another of
spinal cord, and a third of muscle cells. Stimulating the cortex organoid
caused the muscle cells to contract.
But organoids are
far from being miniature brains. For one thing, their neurons remain stunted.
For another, they are not as electrically active as ordinary neurons in a
living brain. “It’s clear that there are a number of limitations to these
models,” Pasca said.
Scientists began
putting organoids in living brains, theorizing that a petri dish limited an
organoid’s development. In 2018, neuroscientist Fred Gage and his colleagues at
the Salk Institute for Biological Studies transplanted human brain organoids
into the brains of adult mice. The human neurons continued to mature as the
mouse brain supplied them with blood vessels.
Since then, Gage
and other researchers have implanted organoids into the back of the brain,
where mice perceive signals from their eyes. When the animals saw pulsing
flashes of white light, the human-organoid neurons responded in much the same
way the mouse’s own cells did, according to a study published online in June
that has not yet been peer-reviewed.
Pasca and his team
were also working on organoid transplants, but they chose to put them into
young rodents rather than adults. A day or two after a rat was born, the
scientists injected an organoid the size of a poppy seed into a region of the
brain called the somatosensory cortex, which processes touch, pain and other
signals from across the body. In rats, the region is especially sensitive to
signals from their whiskers.
The human neurons
multiplied in the rat brain until they numbered about 3 million, making up
about a third of the cortex on one side of the rat brain. Each cell in the
organoid grew six times longer than it would have in a petri dish. The cells
also became about as active as neurons in human brains.
Even more
strikingly, the human organoids spontaneously wired themselves into the rat
brain. They connected not just to nearby neurons, but to distant ones as well.
Those connections
made the human neurons sensitive to the rat’s senses. When the researchers blew
puffs of air over the rat’s whiskers, its human organoid crackled in response.
Pasca and his
colleagues also ran experiments to see how the organoids affected the behavior
of the rats, using a water fountain in their chambers.
After 15 days of
training, the rats learned they could get a drink from the fountain when their
organoid was stimulated. The human organoids were apparently sending messages
to the reward-seeking regions of the rats’ brains.
These
species-blending experiments raise provocative ethical questions. Before
starting the work, Pasca consulted with experts at the Center for Law and the
Biosciences at Stanford, who urged him to pay special attention to the animals’
pain and well-being.
“You’re not just worried
about how many mice are in a cage, or how well they’re fed,” said Henry Greely,
a Stanford law professor. “This is a new kind of thing. You don’t know what you
might see.”
Pasca’s team found
no evidence that the rats experienced pain, became prone to seizures, or
suffered a loss of memory or control of their movements. “It turns out that the
rats tolerate the human graft really well,” Pasca said.
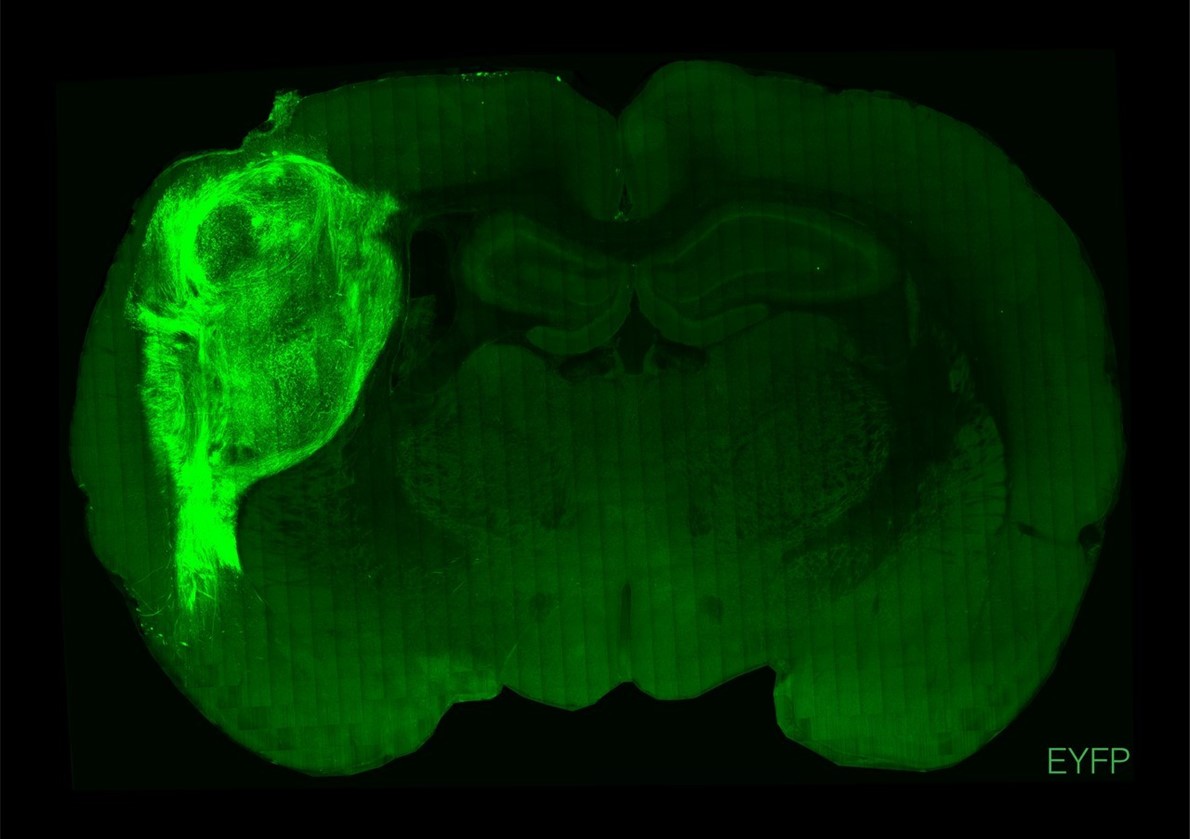 An image provided by Stanford University shows a slice of rat brain with the human cortical organoid in bright green. Scientists have successfully transplanted clusters of human neurons into the brains of newborn rats, a striking feat of biological engineering that may provide more realistic models for neurological conditions such as autism and serve as a way to restore injured brains.
An image provided by Stanford University shows a slice of rat brain with the human cortical organoid in bright green. Scientists have successfully transplanted clusters of human neurons into the brains of newborn rats, a striking feat of biological engineering that may provide more realistic models for neurological conditions such as autism and serve as a way to restore injured brains.
Giorgia Quadrato, a
neurobiologist at the
University of Southern California who was not involved in
the new study, noted that the human organoids did not make the rats more human.
On learning tests, for example, they scored no better than other rats.
“They are rats, and
they stay rats,” Quadrato said. “This should be reassuring from an ethical
perspective.”
But that might not
hold true if scientists were to put human organoids in a close relative of
humans, like a monkey or a chimpanzee. “It would be a good opportunity to set
guidelines to operate in the right ethical framework in the future,” she said.
Pasca said that the
similarity between primates and humans might allow the organoids to grow more
and take on a bigger role in the animal’s mental processes. “It’s not something
that we would do, or would encourage doing,” he said.
Pasca is using the
implanted organoids to study neurological disorders. In one experiment, Pasca’s
team implanted an organoid from a patient with Timothy syndrome on one side of
a rat’s brain and implanted another organoid without the mutation on the other
side.
Both organoids grew
in the rats. But the Timothy syndrome neurons developed twice as many branches
for receiving incoming signals, called dendrites. What’s more, the dendrites
were shorter.
Pasca hopes that he
will be able to observe differences in the way rats behave when they carry
brain organoids from people with autism and other neurological conditions. Such
experiments could help reveal how certain mutations alter the way the brain
works.
Dr Isaac Chen, a
neurosurgeon and organoid researcher at the
University of Pennsylvania who was
not involved in the research, saw another possibility in the new study: the
repair of injuries to human brains.
Chen envisioned
growing brain organoids from the skin of a patient with a damaged cortex. Once
injected into the brain of the patient, the organoid might grow and wire up
with healthy neurons.
“This idea is
definitely out there,” he said. “It’s just a matter of, how do we take
advantage of it, and take it to the next level?”
Read more Odd and Bizarre
Jordan News




